Biopharma Institute's on-line schooling programs consist of immediate obtain after enrollment. Username, password, and directions are emailed to the coed instantly next on the internet enrollment into This system. Study course takers could endeavor the final evaluation any number of moments, as wanted, to attain a passing rating.
Ahead of any processing starts, a Check out ought to be performed and recorded to make certain that the tools and workstation are clear of preceding products and solutions, documents, or materials not necessary to the prepared method and which the products is cleanse and well suited for use.
The community high quality assurance unit has the obligation of guaranteeing by means of organizational steps and auditing that GMP documentation and information systems utilised inside the operational unit are finish and adjust to the relevant GMP demands, and likewise that the requirements from the SOPs are adopted.
Fantastic Documentation Procedures, commonly abbreviated as GDP, confer with a list of recommendations that make sure documents are made and preserved correctly, constantly, and in a managed manner over the pharmaceutical industry.
Although the rationale of a specific action is probably not quickly clear, it may have already been set there as a Check out for another stage of the procedure. Strategies for advancement need to generally be inspired, but tend not to transform strategies with no evaluating the impact on the complete process.
SimplerQMS delivers lifetime science QMS software package with strong document management capabilities, enabling pharmaceutical companies to successfully deal with and Handle documents and data in the course of their lifecycle.
The unit is a priceless products to the maker. It lets to display compliance utilizing the expertise and familiarity with the developer(s); because of their knowledge of The interior processes with the Software, they are able to put together a relatively lean protocol that sufficiently troubles the solution.
Batch production and laboratory Command data of important approach methods ought to be reviewed and authorized by the standard device(s) right before an API batch is released or distributed.
The title of your product, the batch read more range and the quantity of solution for being packed, plus the amount actually acquired and its reconciliation
If I had many printouts (information), all linked to 1 distinct check - Each and every page immediately states Web site one of 1 - is it doable to staple many of the related webpages with each other (dealt with now as 1 doc/attachment), sign the entrance page as the individual it was done by and pagerise the remaining webpages?
In addition to official instruction, a QA individual have to have focus to detail, exceptional conversation and interpersonal skills & capacity to work very well in a very staff.
Starting up products in the storage area ought to be properly labeled. Labels need to click here bear no less than the next data:
Is there an sufficient method in place to guarantee that important procedure adjustments, such as the use of subcontractors as well as their impact on the item, are communicated to The shopper?
Documentation is The true secret to GMP compliance and guarantees traceability of all development, manufacturing, and screening routines. Documentation supplies the route for auditors to evaluate the overall high quality of operations inside an organization and the ultimate products.
 Alana "Honey Boo Boo" Thompson Then & Now!
Alana "Honey Boo Boo" Thompson Then & Now!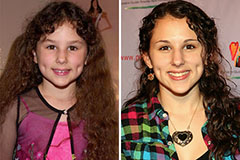 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now!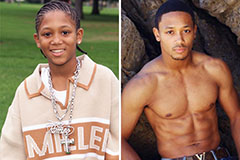 Romeo Miller Then & Now!
Romeo Miller Then & Now! Seth Green Then & Now!
Seth Green Then & Now! Gia Lopez Then & Now!
Gia Lopez Then & Now!